Which Measurement Is Directly Related To The Ph Of The Body
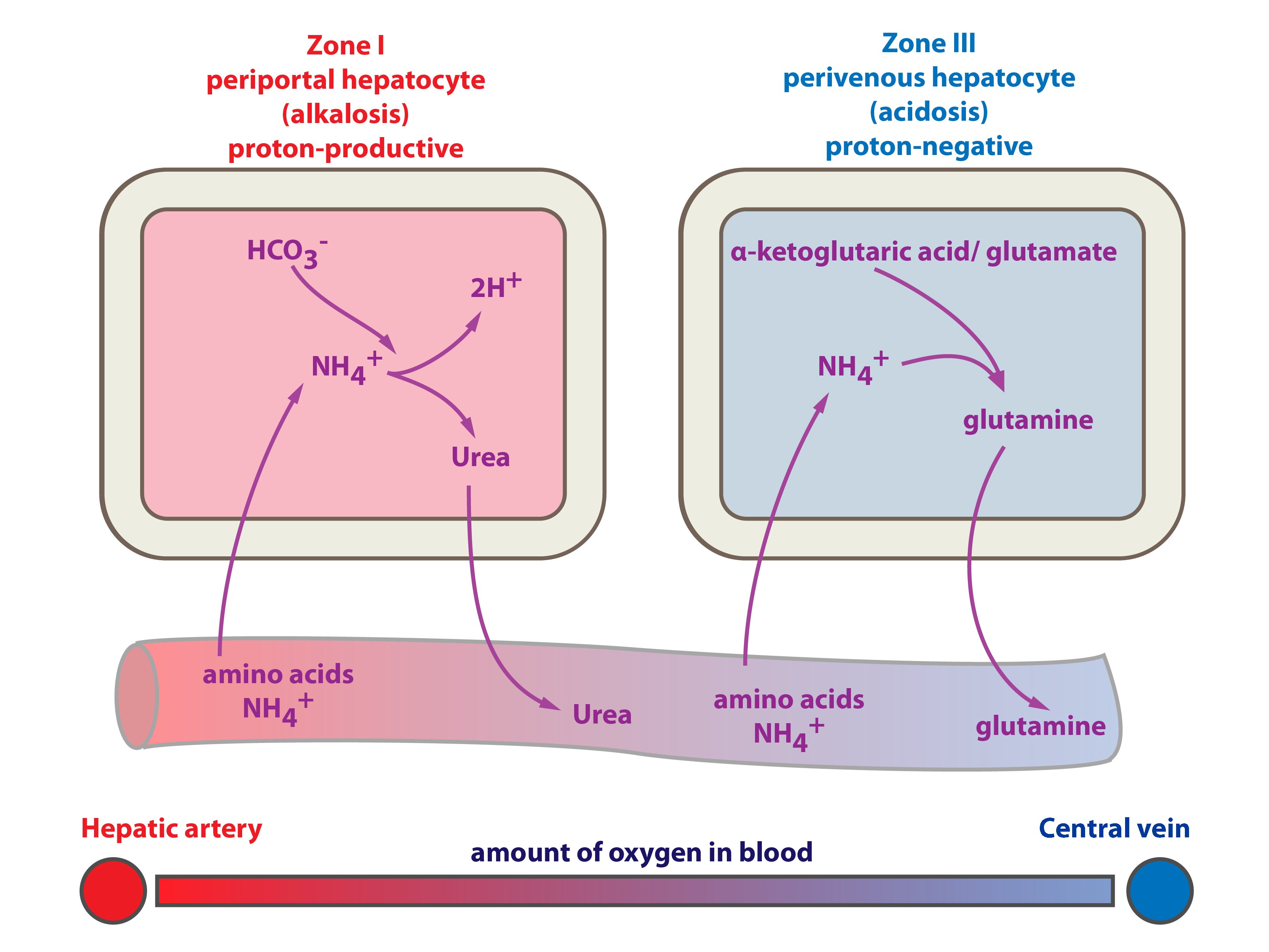
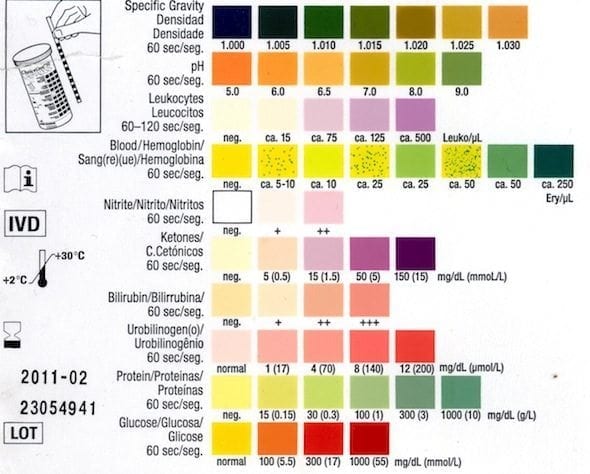
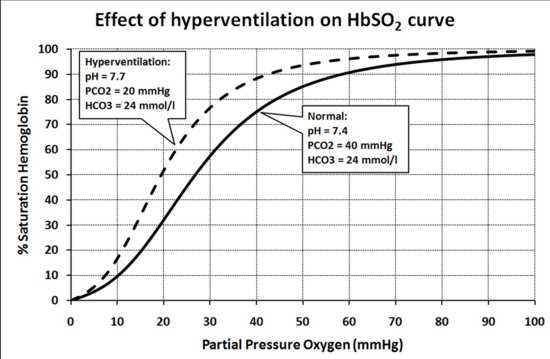
The parts and fluids of the human body have ph values. The chemical balance in a human body is determined due to the ph of blood and other fluids. Ph meter measures acid and base in a fluid. While alkalinity and ph are closely related there are distinct differences. Hence ph is defined as hydrogen ion concentration in fluid. The cell consists of a measuring and reference electrode.
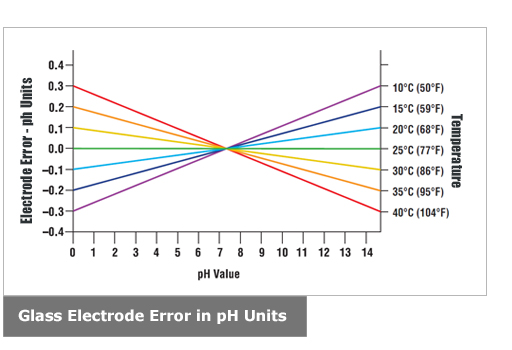
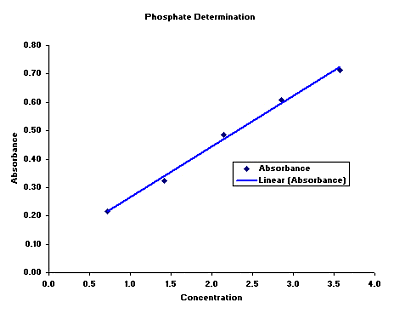
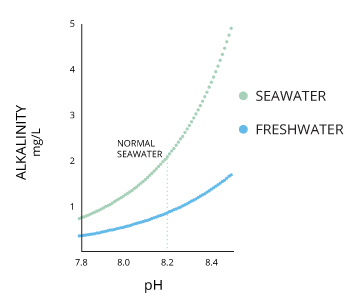
Your ph score represents the amount of acid in your body. The measured area was 96 50 mm and the number of measuring points was 48 25. The alkalinity of water or a solution is the quantitative capacity of that solution to buffer or neutralize an acid. The measured area included the points directly under both the anode and the cathode. For the scientifically inclined ph or power of hydrogen is a measurement of the hydrogen ion concentration in your body. The proportionality constant depends on temperature so a temperature sensor is also necessary.
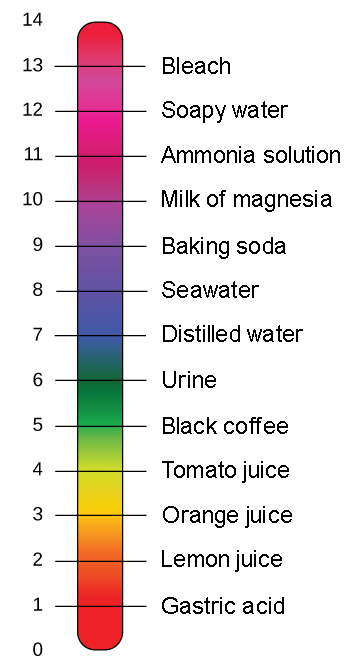
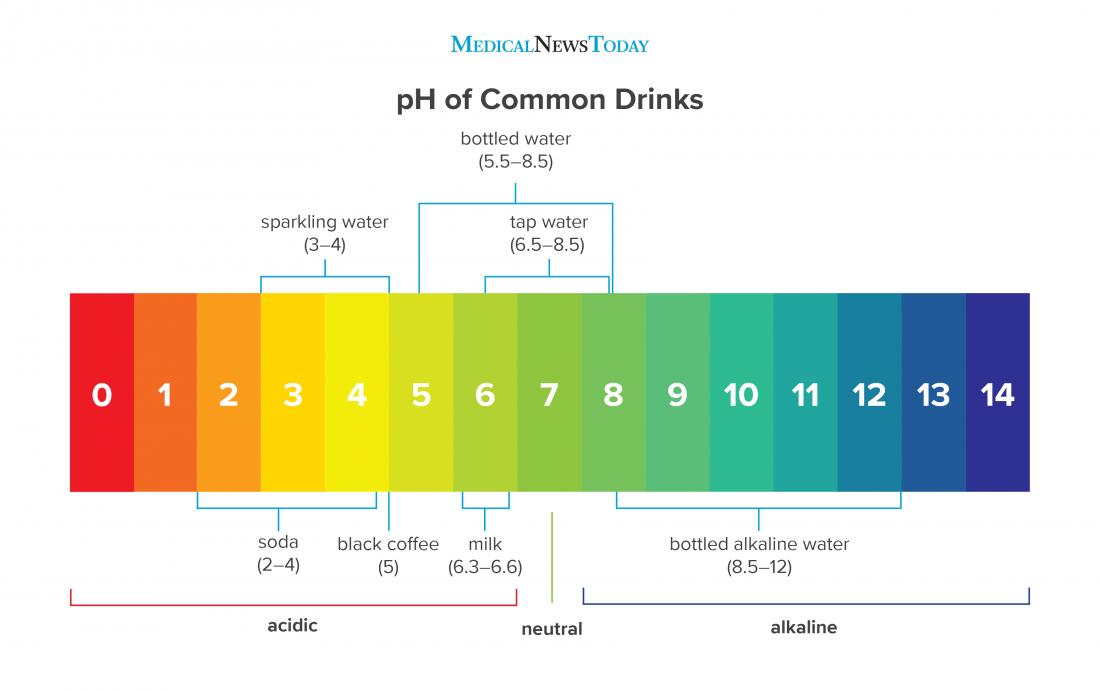
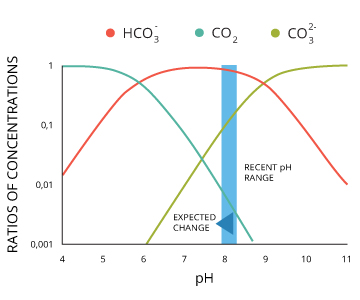
Measurement of ph and related terminology. The voltage between the electrodes is directly proportional to the ph of the test solution. Alkalinity and ph are directly related at 100 air saturation. It is only for very dilute solutions that the ph can be directly. Alkalinity does not refer to alkalis as alkaline does ⁶. While 70 is considered neutral a score below 7 shows that your body is acidic while a score above indicates you have reduced the acidity to a healthy level.
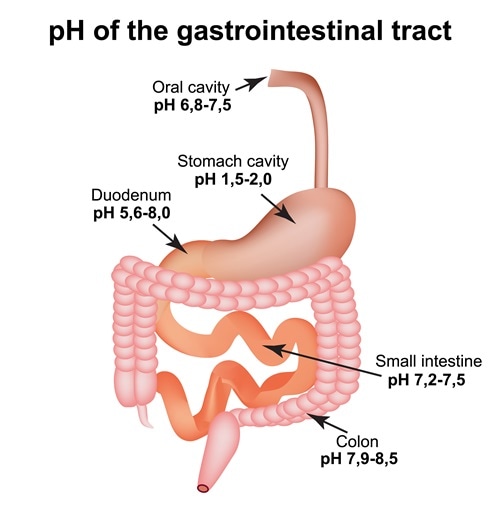
When the solution is neutral it has a ph value of 7 when lesser than 7 it is acidic and more than 7 indicates it is basic solution. Two dimensional ph measurements of electrolyte were carried out at the bottom of an electrolysis cell. Nakao in encyclopedia of food microbiology second edition 2014.
Random Post
- pulkit samrat body measurement
- bus body measurement
- body fat measurement athlete or normal
- mankirt aulakh body measurement
- wwe kaitlyn body measurement
- body fat measurement halifax
- full female body measurement chart
- fitbit body fat measurement
- slm body measurement
- river fox body measurements
- method of body measurement
- aneri vajani body measurement
- body measurement bpm
- body measurement template google sheets
- rajpal yadav body measurement
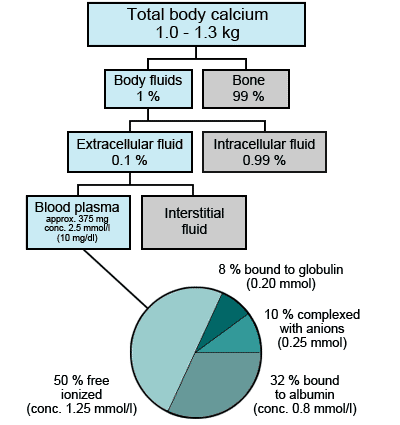
- total body potassium measurement
- where to buy body measurement tape
- hailey bieber body measurement
- dhruv vikram body measurement
- chris evans body measurements
- body measurements free app
- nikita dutta body measurement
- buffbunny body measurements
- nia sharma body measurement
- daniel radcliffe body measurements
- body fat measurement device sri lanka
- safa kabir body measurement
- lisa edelstein body measurement
- seema singh body measurement
- can yaman body measurement
- jacinda ardern body measurement
- jin kazama body measurement
- beachbody challenge measurements
- rimi sen body measurement
- downloadable body measurement chart
- keto body measurement chart
- bra measurement sizes
- jennie body measurement
- teresa giudice body measurement
- shruti hassan body measurement
- sadik hadzovic body measurement
- if my body measurements
- best bollywood actress body measurement
- body measurement chart
- rose mciver body measurement
- alia bhatt body measurement
- what do my body measurements mean
- body measurement outline
- army body fat measurement instructions
- ashanti body measurement